Introduction of ph meter
Definition of ph meter
A pH meter refers to an instrument used to determine the pH value of a solution. The pH meter works on the principle of a galvanic battery. The electromotive force between the two electrodes of the galvanic battery is based on Nerns's law, which is not only related to the properties of the electrodes, but also related to the concentration of hydrogen ions in the solution. There is a corresponding relationship between the electromotive force of the primary battery and the hydrogen ion concentration, and the negative logarithm of the hydrogen ion concentration is the pH value. The pH meter is a common analytical instrument, which is widely used in agriculture, environmental protection and industrial. Soil pH is one of the important basic properties of soil. Factors such as the temperature and ionic strength of the solution to be tested should be considered during the pH measurement.
The principle of ph meter
pH is defined as the negative logarithm of the hydrogen ion concentration in the aqueous solution. Although this sounds complicated, in very simple terms, pH is a number used to quantify the acidity or alkalinity of a solution. The number indicates the number of hydrogen ions that a specific substance can release in the solution. In the pH range, a pH of 7 is considered neutral. Solutions with a pH of 0-7 are considered acidic, and solutions above 7 to 14 are called alkaline solutions. In biological systems, pH is critical. Thanks to the carefully adjusted pH, most of the biomolecules in our body can perform excellent functions. Even in an experimental system, the required pH must be maintained to obtain accurate results. Therefore, in biological experiments, a device called a pH meter is used to carefully monitor pH.
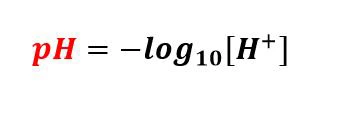
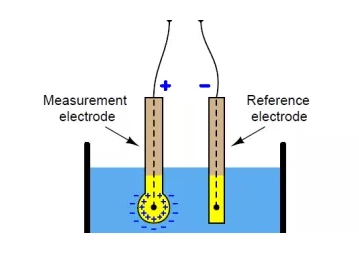
The pH meter is a pH-responsive electrode that measures the activity of hydrogen ions in a solution and transmits this information. The device consists of two glass tubes, each of which contains an electrode, a reference electrode and a sensor electrode. The reference electrode is made of saturated KCl solution, while the sensor electrode contains a buffer solution with a pH of 7, and the silver wire coated with silver chloride is immersed in these two solutions. At the end of the sensor electrode is a bulb made of porous glass coated with silica and metal salt.
To measure the pH of the solution, the pH meter is immersed in the solution. After the bulb of the sensor electrode contacts the solution, the hydrogen ions in the solution will replace the metal ions on the bulb. This substitution of metal ions causes current to flow in the metal wire, which is read by a voltmeter.
The pH meter is one of the most widely used equipment in biological laboratories. Routinely analyze the pH of buffers, solutions and reagents to ensure that the experimental conditions are correct. To ensure accurate readings, the equipment must be calibrated regularly.
Application of PH meter detector
Application of PH meter detector in domestic sewage treatment process

Application of pH meter in electroplating wastewater treatment

Application of Online PH Meter in Industry

Calibration of PH meter



